
We use cookies and similar technologies that are necessary to operate the website. Additional cookies are used to perform analysis of website usage. please read our Privacy Policy
Clinical Trial Management Software Development: Benefits, Features, Process

Clinical trials are crucial for advancing medicine by testing new treatments and therapies. They help improve healthcare by finding safer and better ways to treat diseases and help patients. However, organizing clinical trials is complex. It involves managing logistics, following rules, and handling lots of data.
That’s why, healthcare organizations have started using Clinical Trial Management Software (CTMS) is like a helpful guide that helps researchers and doctors guide through these processes.
CTMS isn’t just about making paperwork easier. It changes how research is done. By automating tasks, ensuring rules are followed, and helping teams work together, CTMS makes clinical trials more efficient and accurate.
In this blog, we will explore what CTMS is in detail. We’ll explore the real-life use cases of clinical trial management software and a step-by-step guide to developing a custom clinical trial management software for your healthcare organization.
Let’s first understand:
What is Clinical Trial Management Software?
Clinical Trial Management Software (CTMS) is a specialized tool used by researchers and pharmaceutical companies to manage and streamline the process of conducting clinical trials. It helps organize and track all aspects of a trial, from participant recruitment and scheduling to data collection and analysis.
Essentially, CTMS makes it easier to oversee and manage the complex logistics and data involved in clinical research, ensuring trials are conducted efficiently and according to regulatory standards.
Key Statistics On The Clinical Trial Management System (CTMS)
- The global CTMS market was valued at USD 1.85 billion in 2023 and is expected to grow at a rate of 14.65% annually from 2024 to 2030, reaching USD 4.73 billion by 2030.
- In 2021, the CTMS market was around USD 1.2 billion and is projected to grow at a rate of 11.4% annually from 2022 to 2030.
- The enterprise-based CTMS market is forecasted to grow by 11.4% annually from 2022 to 2030.
- North America led the CTMS market in 2023, accounting for about 50% of the market share, driven by the presence of major companies and increased technology adoption in research and development (R&D).
The CTMS market is experiencing significant growth driven by the increasing complexity of clinical trials, the need for streamlined operations, and rising R&D investments by pharmaceutical and biotech companies globally.
Benefits Of Clinical Trial Management Software
Using Clinical Trial Management Software (CTMS) can revolutionize the way you manage your clinical trials. Here’s how it can benefit you:
1. Simplified Trial Planning and Execution
One important benefit of CTMS is its capability to streamline the entire process of clinical trials by reducing essential trial information into a single accessible platform. This includes protocols, investigator details, participant demographics, and regulatory requirements, all digitized for improved accessibility and efficiency.
This digital transformation allows researchers and trial managers to effortlessly create timelines, assign tasks, and track progress in real-time. Streamlining these processes reduces administrative hassles, minimizes errors, and accelerates the start of trials, ensuring smoother journeys for everyone involved.
2. Improved Participant Recruitment and Retention
Recruiting and retaining participants in clinical trials can be a real challenge. CTMS steps in to help with integrated participant databases and recruitment tools.
Through automated screening, it matches eligible candidates to trial criteria swiftly, making recruitment smoother. Also with the benefit of personalized communication features, it promotes trust and commitment among participants, ensuring they stay engaged throughout the trial journey.
3. Effective Data Management and Analysis
In successful clinical trials, effective data management is crucial. CTMS improves this by offering secure, centralized repositories that meet strict regulatory standards, such as those set by the FDA.
Another benefit of clinical trial management software is researchers gain real-time access to trial data, allowing them to monitor safety parameters, track adverse events, and perform interim analyses promptly.
Integrated advanced analytics tools within CTMS provide insightful reporting, empowering researchers to make data-driven decisions that improve trial outcomes and accelerate regulatory submissions.
4. Meeting Regulatory Requirements
Ensuring clinical trials run smoothly and meet regulatory standards is important. One significant benefit of CTMS is its role in automating paperwork, securely managing records, and promoting communication with regulators.
By establishing clear guidelines and ensuring data accuracy, CTMS reduces the risks associated with non-compliance. This proactive approach prepares trials for regulatory inspections and expedites the approval process.
With CTMS, clinical research teams can guide regulatory requirements more efficiently, promoting a streamlined path to faster approvals and ultimately advancing medical innovation.
5. Cost Saving
Beyond improving efficiency, CTMS offers additional benefits by contributing to cost savings and optimizing resources throughout the clinical trial lifecycle. By automating manual tasks and minimizing administrative burdens, organizations can strategically allocate resources, thereby maximizing research budgets and accelerating the introduction of groundbreaking therapies. This not only streamlines operations but also promotes a more agile and effective approach to medical research, ultimately driving forward progress in healthcare.
Now that we have understood the benefits of clinic trial management software, let’s explore the features behind these advantages.
Key Features Of A Clinic Trial Management Software
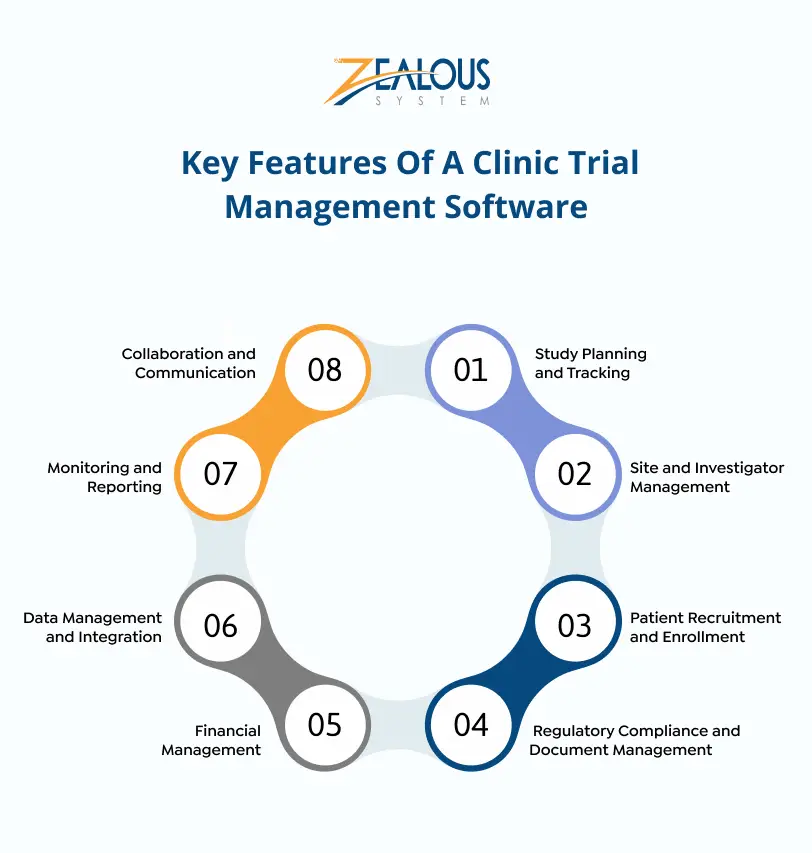
Clinical trials are crucial for developing new medical treatments. However, managing them involves lots of tasks and rules. That’s where Clinical Trial Management Software (CTMS) comes in. Here are the main features of some of the best clinical trial management software that make it important for researchers and organizations in clinical trials.
1. Study Planning and Tracking
One of the primary features of a CTMS is to simplify the planning and tracking of clinical studies. This feature of Clinical Trial Management Software allows researchers to create detailed study plans, including timelines, milestones, and deliverables.
By providing a centralized platform for tracking progress, a CTMS ensures that all team members are on the same page, helping to prevent delays and ensure that the study stays on track.
2. Site and Investigator Management
Managing multiple trial sites and investigators can be a logistical nightmare. CTMS simplifies this process by offering tools to manage site information, investigator credentials, and communication. This feature of CTMS helps ensure that all sites adhere to the same protocols and standards, improving overall study consistency and reliability.
3. Patient Recruitment and Enrollment
Recruiting and enrolling patients is a critical phase in any clinical trial. CTMS offers power tools to manage patient recruitment, screening, and enrollment. This feature of CTMS can significantly reduce the time and effort required to find suitable candidates and ensure they meet the study’s criteria.
4. Regulatory Compliance and Document Management
Compliance with regulatory standards is important in clinical trials. CTMS provides a centralized repository for all regulatory documents, ensuring that all necessary approvals and submissions are tracked and managed efficiently. This feature helps organizations maintain compliance with regulatory bodies like the FDA, EMA, and other international agencies.
5. Financial Management
Clinical trials involve significant financial investments. A CTMS includes features for budgeting, forecasting, and tracking expenses, helping organizations manage their financial resources effectively. This feature also supports grant management, contract tracking, and payment processing, ensuring that all financial aspects of the trial are handled smoothly.
6. Data Management and Integration
The ability to collect, store, and analyze data is crucial in clinical trials. CTMS offers robust data management tools that support the integration of various data sources, including Electronic Data Capture (EDC) systems, Laboratory Information Management Systems (LIMS), and more. This feature ensures that all trial data is accurate, up-to-date, and readily accessible for analysis.
7. Monitoring and Reporting
Effective monitoring and reporting are essential for the success of clinical trials. CTMS provides tools for real-time monitoring of trial activities and comprehensive reporting capabilities. This feature allows researchers to generate custom reports and dashboards, providing valuable insights into trial performance and enabling data-driven decision-making.
8. Collaboration and Communication
Clinical trials often involve collaboration between various stakeholders, including researchers, sponsors, regulatory bodies, and patients. CTMS facilitates effective communication and collaboration through integrated messaging systems, shared calendars, and document collaboration tools. This feature ensures that all stakeholders are aligned and can work together efficiently.
Best Use Cases of Best Clinical Trial Management Software
Clinical trial management software (CTMS) plays a crucial role in advancing medical research and improving patient outcomes. From patient recruitment to data analysis, CTMS offers a complete solution that impacts every stage of the trial lifecycle. Here are some of the main use cases of Clinic Trial management software:
1. Recruitment Management
CTMS simplifies patient recruitment by automating tasks like screening eligibility criteria and managing patient data. Using advanced algorithms and patient databases, researchers can identify suitable candidates more efficiently, reducing recruitment timelines and ensuring trials are completed on schedule.
2. Quality Assurance
Another use case of CTMS is in maintaining protocol compliance is essential for the success and credibility of clinical trials. CTMS allows researchers to build, manage, and enforce study protocols, ensuring adherence to regulatory standards. Real-time data capture and monitoring tools help maintain data accuracy and quality, minimizing errors during the trial.
3. Site Management
CTMS promotes seamless communication and collaboration between investigators, sponsors, and clinical research coordinators (CRCs) across multiple sites. This feature of centralized document management, protocol updates, and task tracking helps in improving operational efficiency and coordination, improving overall trial management.
4. Financial Management
Managing resources and budgets well is crucial for clinical trials to succeed. CTMS (Clinical Trial Management System) helps by providing tools for planning budgets, tracking expenses, and allocating resources. Its real-time reporting and analytics feature also help stakeholders make smart financial decisions, ensuring that trials stay within their budget limits.
5. Regulatory Compliance Management
Making sure clinical research follows regulations is crucial for patient safety and data reliability. CTMS (Clinical Trial Management System) helps by automating tasks like managing regulatory documents, tracking submissions, and preparing for audits.
This simplifies meeting standards such as Good Clinical Practice (GCP) and FDA requirements. The software’s reporting features also help generate regulatory reports quickly, making it easier to get approvals for trials.
Software Development Trends In Clinical Trial Management Software

Technology is bringing new levels of efficiency and effectiveness. Here’s how the latest trends in clinical trial management software are changing the way trials are conducted and managed:
Integration of AI and Machine Learning
One of the most in-demand software development trends in clinical trial management software is the use of Artificial Intelligence solutions and Machine Learning services which are leading the charge in transforming clinical trial management. These technologies automate tasks, predict patient outcomes, and optimize trial protocols by analyzing large datasets. They identify trends and anomalies, speeding up decision-making and cutting costs.
Real-Time Data Analytics
Now, researchers can monitor trial progress, patient recruitment, and safety outcomes instantly. Advanced analytics is another beneficial trend in clinical trial management software which provides clear insights, helping stakeholders make quick decisions and adjust trial protocols as needed. This agility improves trial efficiency and success rates.
Patient-Centric Solutions
All the best clinical trial management software focuses on improving the participant experience. With easy-to-use interfaces, mobile apps, and remote monitoring, these innovations empower patients to actively engage in trials. This approach boosts retention rates and ensures accurate data collection for better trial outcomes.
Blockchain Technology for Data Security
Protecting data integrity is crucial in clinical trials. Blockchain technology is an important trend in CTMS which creates a secure, transparent ledger for trial data, enhancing privacy and compliance. This builds trust with users and regulators, ensuring reliable and safe trials.
Telemedicine and Virtual Trials
Telemedicine and virtual trial capabilities have emerged as game-changers in clinical research. These technologies allow remote patient monitoring software , virtual visits, and electronic data capture, reducing the dependency on in-person visits. By using telemedicine, researchers can conduct trials more efficiently, expand participant recruitment globally, and improve collaboration among research teams across borders.
The adoption of virtual trials not only minimizes patient burden but also improves accessibility to clinical studies, making participation more convenient and inclusive.
Integration with Electronic Health Records (EHR)
Integration with EHR systems has streamlined data exchange between healthcare providers and research teams. Integration is a significant software development trend in CTMS which allows seamless access to patient medical histories, laboratory results, and treatment records directly within the CTMS. By using EHR data, researchers can improve data quality, reduce redundancy in data entry, and speed up the initiation and execution of clinical trials.
Regulatory Compliance and Risk Management
Ensuring regulatory compliance is crucial in clinical trials, and modern CTMS platforms integrate powerful features to adhere to international standards such as Good Clinical Practice (GCP) and FDA regulations. These platforms include built-in risk management tools that identify and mitigate potential risks throughout the trial lifecycle, ensuring data integrity and safeguarding patient safety.
Mobile Applications and Wearable Devices
The integration of mobile applications and wearable devices has transformed how clinical trial data is collected and monitored. These technologies allow real-time gathering of health data from participants, including vital signs, activity levels, and medication adherence. By linking with CTMS platforms, researchers can capture objective data remotely, providing deeper insights into patient behavior and health outcomes during trials.
By staying informed about these healthcare technologies trends in CTMS, you can enhance your Clinical Trial Management System, improve trial efficiency, and achieve better outcomes.
Development Process of Clinical Trial Management Software: Step By Step Guide
Developing the best clinical trial management software involves a systematic approach to ensure that the software meets the specific needs of clinical research organizations. Here’s a detailed step-by-step guide:
Phase 1: Conceptualization and Planning
Like any innovative project, developing CTMS starts with a clear vision. Here you will need to collaborate closely with clinical research experts to outline the software’s functionalities. This phase is all about asking the right questions:
- What specific challenges do researchers face?
- How can technology simplify data collection and analysis?
- What regulatory standards must the software meet?
By aligning technology with real-world needs, you can ensure the software addresses critical pain points in clinical trial management.
Step 2: Requirements Gathering
Next, gather detailed requirements from all stakeholders. This involves conducting interviews, surveys, and workshops with clinical researchers, sponsors, regulatory bodies, and other users. Understanding their needs and expectations is crucial for developing a comprehensive requirements document that guides the design and development process.
Step 3: Design and Prototyping
With a roadmap in hand, it’s time to bring your ideas to life. UX/UI designers create intuitive interfaces that prioritize user experience. Prototyping allows you to gather early feedback and make iterative improvements. This step ensures the design meets users’ needs and expectations, emphasizing clarity and efficiency.
Step 4: Development
After finalizing your design, the next step is development. Here, backend development focuses on creating the server, database, and application logic, while frontend development designs the user interface to be responsive and easy to use.
Software integration is also crucial, linking your CTMS with systems like Electronic Data Capture (EDC) systems, regulatory databases, and clinical trial registries. This ensures smooth data flow and operational efficiency across your platforms.
Step 5: Testing
Thorough testing is critical to ensure your CTMS functions correctly and reliably. Conduct unit testing, integration testing, system testing, and user acceptance testing to identify and resolve any issues. Rigorous testing ensures the software is robust, secure, and ready for deployment in real-world clinical trial settings.
Step 6: Deployment
Once testing is complete and everything works well, it’s time to launch your CTMS. This means preparing the live environment, moving over your data, and setting everything up for real-world use. It’s important to train users thoroughly so they can use the system with confidence. Keep a close watch on the launch to quickly fix any problems that come up.
Step 7: Maintenance and Support
The final step is ongoing maintenance and support. Regularly update your CTMS to keep it compliant with changing regulations and to incorporate new features and improvements. Provide continuous support to users, addressing any issues promptly and ensuring the system remains reliable and efficient over time. Your commitment to maintenance and support ensures the long-term success of the CTMS.
By following these steps, you can develop the best clinical trial management software that streamlines clinical trial processes, ensures compliance, and improves data management and reporting capabilities.
Conclusion
In conclusion, it’s clear that clinical trial management software is more than just a tool, it’s a key driver of efficiency and innovation in healthcare research.
By using advanced technology and customized software features, these solutions help healthcare professionals streamline their work and speed up medical progress.
Developing CTMS isn’t just about building software; it’s about investing in a healthier future for everyone. Let’s develop healthcare software together at Zealous System that not only manages trials but also changes lives and shapes a better future for healthcare.
We are here
Our team is always eager to know what you are looking for. Drop them a Hi!
Umang Baraiya
I am currently working as a business analyst at Zealous System. I am experienced in working with stakeholders and managing project requirements, Documentation of requirements, and planning of product backlog.
Table of Contents
×


Comments